8. What is the cloud point of surfactant? What is its important role in dyeing and finishing production and auxiliary production?
Answer The chemical structural formula of polyethylene glycol nonionic surfactant can be written as~OCH2CH2OCH2CH2 OCH2CH2~, the molecular chain is linear. Judging from its molecular arrangement, it is zigzag-shaped in anhydrous state, and mainly zigzag-shaped in aqueous solution, as shown in the figure below. 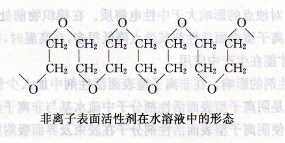 The hydrophilic oxygen atom is placed on the outside of the chain, and the hydrophobic CH2One is located on the inner side, and the whole is just like a hydrophilic base. The oxygen atoms in the ether bond and the hydrogen in the water molecules combine with weak chemical force to form a hydrogen bond, thus increasing the solubility in water. However, the bond between the oxygen atom of the ether bond and the water molecule is not strong because the hydrogen bond is relaxed, and the bond energy of the hydrogen bond is about 29.26kJ/mol. When an aqueous solution of polyethylene glycol nonionic surfactant is heated, as the temperature increases, the bound water molecules gradually detach, and its hydrophilicity gradually weakens. Then, it rapidly becomes insoluble in water, so that the initially transparent solution turns into a white turbid emulsion, and can return to a transparent solution after cooling. The temperature at which this nonionic surfactant solution first becomes cloudy is called the “cloud point”. This is particularly important data, related to surfactant properties. Polyethylene glycol nonionic surfactants are soluble in water below the cloud point and insoluble in water above the cloud point. There are roughly four factors that affect cloud point.
The hydrophilic oxygen atom is placed on the outside of the chain, and the hydrophobic CH2One is located on the inner side, and the whole is just like a hydrophilic base. The oxygen atoms in the ether bond and the hydrogen in the water molecules combine with weak chemical force to form a hydrogen bond, thus increasing the solubility in water. However, the bond between the oxygen atom of the ether bond and the water molecule is not strong because the hydrogen bond is relaxed, and the bond energy of the hydrogen bond is about 29.26kJ/mol. When an aqueous solution of polyethylene glycol nonionic surfactant is heated, as the temperature increases, the bound water molecules gradually detach, and its hydrophilicity gradually weakens. Then, it rapidly becomes insoluble in water, so that the initially transparent solution turns into a white turbid emulsion, and can return to a transparent solution after cooling. The temperature at which this nonionic surfactant solution first becomes cloudy is called the “cloud point”. This is particularly important data, related to surfactant properties. Polyethylene glycol nonionic surfactants are soluble in water below the cloud point and insoluble in water above the cloud point. There are roughly four factors that affect cloud point. 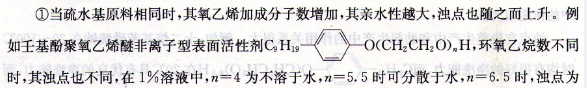
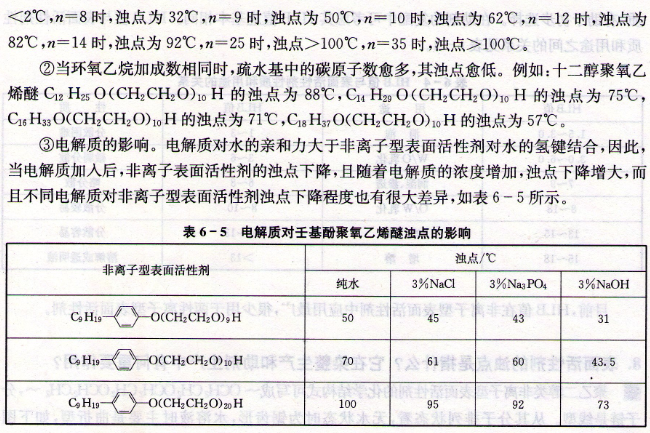 TheabovetableshowsthatNaOHhasagreaterimpactoncloudpointthanneutralelectrolyte.Inthepre-treatmentofcottonfabrics,itneedstobecarriedoutinalkalineorstrongalkalisolutions.Thecloudpointofthenon-ionicsurfactantisgreatlyreduced.Athightemperatures,theeffectofthesurfactantisoftenlost,soeffortsshouldbemadetoincreasethecloudpoint.,canbeappliedinproduction.④Theinfluenceofionicsurfactants.Addingasmallamountofanionicsurfactanttoanonionicsurfactantcanincreaseitscloudpoint.Thisisduetotheinteractionbetweenthehydrophobicgroupsintheanionicsurfactantmoleculesandthehydrophobicgroupsofthenonionicsurfactant.Gravitypromotestheinsertionofanionicsurfactantmoleculesintomicellesormoleculesofnon-ionicsurfactantsonthemicellesandinterfaceadsorptionlayers,causingthesurfaceoftheformedmicellestointroducechargesandgenerateelectrostaticinteractions.Astheelectricpotentialincreases,themutualrepulsionbetweenmicellesalsoincreases,whichhinderstheformationoftheaggregationphaseandmakestheadsorptionfilmmoresolid.Theeffectoftemperatureonitisweak,andthecloudpointisincreased.It weakens significantly at 100℃ because it has exceeded its own cloud point. At the same time, above the cloud point, the stability of many emulsions decreases, making it difficult to obtain good emulsification effects. For another example, when non-ionic surfactants are used as leveling agents for high-temperature and high-pressure coloring of disperse dyes, due to the high coloring temperature, the cloud point of the non-ionic surfactants has been exceeded. At this time, the lipophilic dyes are insoluble in the It gathers in the surfactant droplets of water and is adsorbed to the fabric fibers to form spots. If a properly selected anionic surfactant is added, the cloud point of the nonionic surfactant can be increased to above 100°C.
TheabovetableshowsthatNaOHhasagreaterimpactoncloudpointthanneutralelectrolyte.Inthepre-treatmentofcottonfabrics,itneedstobecarriedoutinalkalineorstrongalkalisolutions.Thecloudpointofthenon-ionicsurfactantisgreatlyreduced.Athightemperatures,theeffectofthesurfactantisoftenlost,soeffortsshouldbemadetoincreasethecloudpoint.,canbeappliedinproduction.④Theinfluenceofionicsurfactants.Addingasmallamountofanionicsurfactanttoanonionicsurfactantcanincreaseitscloudpoint.Thisisduetotheinteractionbetweenthehydrophobicgroupsintheanionicsurfactantmoleculesandthehydrophobicgroupsofthenonionicsurfactant.Gravitypromotestheinsertionofanionicsurfactantmoleculesintomicellesormoleculesofnon-ionicsurfactantsonthemicellesandinterfaceadsorptionlayers,causingthesurfaceoftheformedmicellestointroducechargesandgenerateelectrostaticinteractions.Astheelectricpotentialincreases,themutualrepulsionbetweenmicellesalsoincreases,whichhinderstheformationoftheaggregationphaseandmakestheadsorptionfilmmoresolid.Theeffectoftemperatureonitisweak,andthecloudpointisincreased.It weakens significantly at 100℃ because it has exceeded its own cloud point. At the same time, above the cloud point, the stability of many emulsions decreases, making it difficult to obtain good emulsification effects. For another example, when non-ionic surfactants are used as leveling agents for high-temperature and high-pressure coloring of disperse dyes, due to the high coloring temperature, the cloud point of the non-ionic surfactants has been exceeded. At this time, the lipophilic dyes are insoluble in the It gathers in the surfactant droplets of water and is adsorbed to the fabric fibers to form spots. If a properly selected anionic surfactant is added, the cloud point of the nonionic surfactant can be increased to above 100°C.
AAADFGTEHTRY
Disclaimer:
Disclaimer: Some of the texts, pictures, audios, and videos of some articles published on this site are from the Internet and do not represent the views of this site. The copyrights belong to the original authors. If you find that the information reproduced on this website infringes upon your rights, please contact us and we will change or delete it as soon as possible.
AA





